Vegetable oils used for deep frying and biodiesel production are susceptible to oxidation and degradation when subjected to high-temperature processes. Antioxidants can protect oils from peroxidation, depending on factors like oil composition, antioxidant chemical structure, temperature, and impurities.
Researchers at the Department of Chemistry G. Ciamician at the University of Bologna (IT) have described their method which presents a straightforward approach to measuring the stability of oils at elevated temperatures and to studying the efficacy of antioxidants.
Figure 1
 All they needed was a Pyroscience oxygen sensor and glassware normally present in an organic chemistry laboratory. Oxidation tendency and antioxidant effectiveness was accurately determined by measuring oxygen consumption in the headspace with the Pyroscience FireSting-O2 with robust oxygen probe.
All they needed was a Pyroscience oxygen sensor and glassware normally present in an organic chemistry laboratory. Oxidation tendency and antioxidant effectiveness was accurately determined by measuring oxygen consumption in the headspace with the Pyroscience FireSting-O2 with robust oxygen probe.
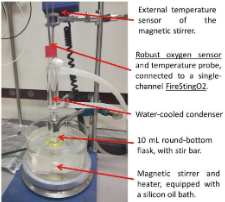
Oil oxidation is performed at 130 °C in a 10 mL round-bottom flask surmounted by a water-cooled refrigerator. To measure the oxygen consumption, the optical oxygen meter and the thermometer are introduced on the top of the refrigerator through a silicone septum ensuring sealing of the apparatus (see Fig. 1). The samples, consisting of 1 or 2 mL of oil, pure or dissolved in a suitable high-boiling solvent, are vigorously stirred by an olive-shaped stir bar and are heated to the required temperature (up to 180 °C) by a silicone oil bath. The glass condenser maintains the probe tip at about 30 °C. The amount of O2 consumed (in mmol) is calculated from the concentration of O2 in the headspace and the internal volume of the apparatus.
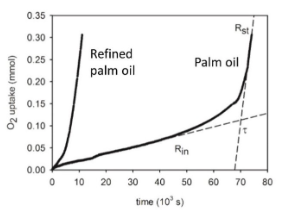
Figure 2.
 As the reaction tends to slow down at low O2 levels, if needed the vessel can be opened, fluxed with fresh air, and closed again to continue the data collection. In Fig. 2, the O2 uptake at 130 °C for two palm oils with different purification degree is reported. An induction period due to the presence of antioxidants can be noticed in the unrefined palm oil sample.
As the reaction tends to slow down at low O2 levels, if needed the vessel can be opened, fluxed with fresh air, and closed again to continue the data collection. In Fig. 2, the O2 uptake at 130 °C for two palm oils with different purification degree is reported. An induction period due to the presence of antioxidants can be noticed in the unrefined palm oil sample.
In conclusion, this method represents an easy and accessible way to measure the stability of oils at high temperature and to study the efficacy of antioxidants. All that is needed is an O2 probe and glassware normally present in an organic chemistry laboratory.
To find out more, please contact our technical sales team.
Contact us




